Tritium
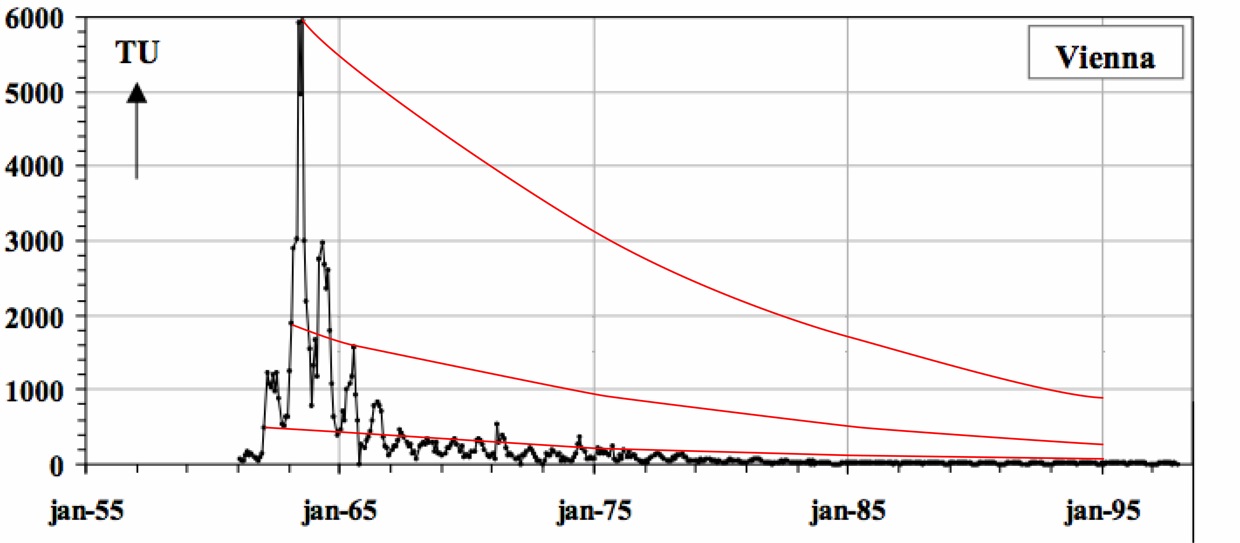
Natural production of tritium is very small compared to the amount of tritium released during the atmospheric atomic bomb testing conducted in the late 1950’s through mid 1960’s. Meteoric tritium levels are naturally 5 TU (tritium units), which is equivalent to 5 tritium atoms in 1018 hydrogen atoms. However, atmospheric atomic bomb testing bolstered the concentration of tritium in the atmosphere resulting in significant global tritium spikes reaching close to 4000 TU in the northern hemisphere. Weather stations have monitored the concentrations of tritium in precipitation giving a global record of tritium concentrations. Because tritium is radioactive and decays with a half-life of 12.4 years to the stable isotope helium-3, the concentrations of tritium in water samples can be measured and the age and original concentrations of tritium can be determined. As illustrated in the image below, a given tritium concentration can lead to non-unique ages. Therefore, the measurement of tritium alone is only appropriate to obtain age ranges, or to distinguish pre-1950's waters from post-1950's waters.
Tritium analysis by helium ingrowth begins with the degassing of water samples within a stainless steel flask. The flask is attached to a high-vacuum, and the samples are agitated to help extract dissolved gases. Four tritium samples can be degasses simultaneously, leaving less than 0.01% gas remaining. After degassing, the samples are sealed and allowed to sit for 6 to 12 weeks while tritium decays and helium-3 grows. The extraction line is also used for extracting dissolved gases from copper tube samples.

The extraction line between sample-runs
After the samples have had sufficient time to decay, the levels of helium-3, which directly correlate to the amount of tritium decayed, is measured using a Mass Analyzers Products – Model 215-50 Magnetic Sector Mass Spectrometer. The MS uses a magnet to deflect ions based on their mass/charge ratios. Two collector are used, one for measuring 3He and one for 4He. An electron multiplier is used to measure low-abundance 3He, while a Faraday cup measures more abundant 4He.

Magnetic Sector-Field Mass Spectrometer
The mass spectrometry system line design is based upon the design described in “A
Static Mass Spectrometer With Triple Collection For Nitrogen and Neon Isotopes”, Scripps
Oceanographic Institute, S.I.O Reference Series # 93-11, September 1993. Atmospheric
gas components are measured and removed using SAES getter technology (see SAES) and cryogenics. The system is held at high vacuum using a combination of rough pumps,
turbo pumps, getters and ion pumps. The entire system is computer controlled through
two computer systems utilizing National Instruments hardware and software.
Gas Standards - A tank of “dry” atmosphere is prepared as a daily standard. All Analytical calibrations are made using this standard. In additional, air equilibrated water samples, collected at different temperatures are analyzed as blind standards. Typically, four standards are analyzed for six unknown samples in a given day. The daily schedule is: two standards are run in the morning to ensure proper communication and re-center the peaks, 3 unknown samplers are run followed by a standard. If the standard is acceptable, three more samples are analyzed which are followed by a closing standard. In addition, one air equilibrated water sample is analyzed for approximately every ten unknown samples.
Data analysis – During an analysis, all pertinent information is collected by computer and imported to an Excel worksheet. Three files are produced for every one analysis. On a daily basis, these files are “compiled” into a single workbook using Visual Basic code developed in our lab. This code incorporates the daily standard runs and produces a final sample report. These reports are then reviewed by hand and used in the age calculation process.
